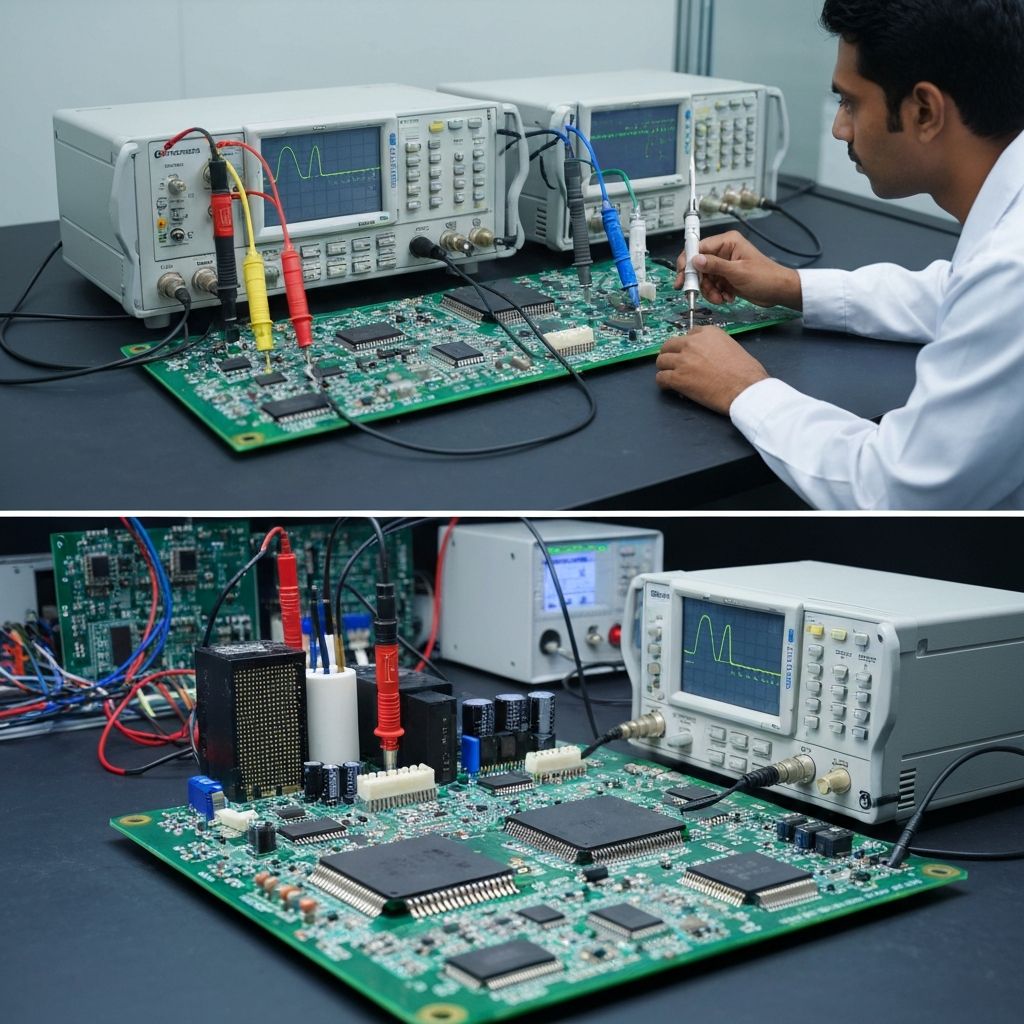
Why Wire Harness Quality Matters in Medical Devices
In medical equipment — patient monitors, diagnostic devices, imaging systems, lab instruments — wiring failures are not just inconvenient; they can affect patient safety. That’s why wire harnesses for medical devices must meet stringent requirements for:
- Reliability and durability
- Electrical safety and insulation
- Biocompatibility (where applicable)
- Regulatory compliance (IEC, ISO, FDA guidelines, etc.)
Key Design Considerations
1. Material Selection
Insulation materials must be selected based on flexibility, temperature range, chemical resistance, and safety ratings. Medical-grade cables may require:
- Low-smoke, halogen-free materials
- Biocompatible jackets for patient-contact devices
- High-flex cables for repetitive motion applications
2. Connector Choices
Connectors should provide secure mating, easy handling, and clear keying to avoid mis-connections in clinical environments. Consider:
- Locking mechanisms (latches, bayonet types)
- Color-coding or mechanical keying for ports
- IP-rated connectors for wash-down or outdoor use
3. EMI/EMC Considerations
Medical devices often coexist with multiple electronic systems in hospitals. Good harness design helps minimize interference by:
- Using shielded cables where needed
- Separating power and signal lines
- Maintaining proper grounding and shielding practices
Manufacturing and Quality Control
For medical wire harnesses, consistent processes and documentation are essential. Professional EMS providers implement:
- Work instructions and process validation
- Crimp pull-force testing and continuity checks
- Insulation resistance and hipot testing
- 100% visual inspection and lot traceability
Regulatory and Documentation Requirements
Many medical projects require detailed documentation such as:
- Material declarations and certificates
- Process validation reports
- Change control and revision history
Working with an EMS partner familiar with medical quality systems (ISO 13485, etc.) makes compliance significantly easier.
Conclusion
Wire harnesses for medical devices demand a careful balance of engineering, materials, process control, and regulatory awareness. By choosing the right design approach and manufacturing partner, you can ensure that your medical products remain safe, reliable, and compliant throughout their lifecycle.